This post is part of our series on key aspects of the final HITECH omnibus rule published by the U.S. Department of Health and Human Services (HHS) in the Federal Register on January 25, 2013. Previous posts are available here. The regulations are effective March 26, 2013, but covered entities and business associates have until September 23, 2013, to comply with most new requirements.
The final HITECH omnibus rule requires covered entities to add several new provisions to the Notice of Privacy Practices (“NPP”) that they distribute to patients and beneficiaries. Generally, an NPP describes how the covered entity may use and disclose protected health information (“PHI”), an individual’s rights with respect to PHI (e.g., the right to access PHI and request restrictions on uses and disclosures), and the covered entity’s legal duties with respect to PHI (e.g., the duty to abide by the terms of the NPP).Continue Reading HITECH Update #8: New Requirements for HIPAA Notices of Privacy Practices
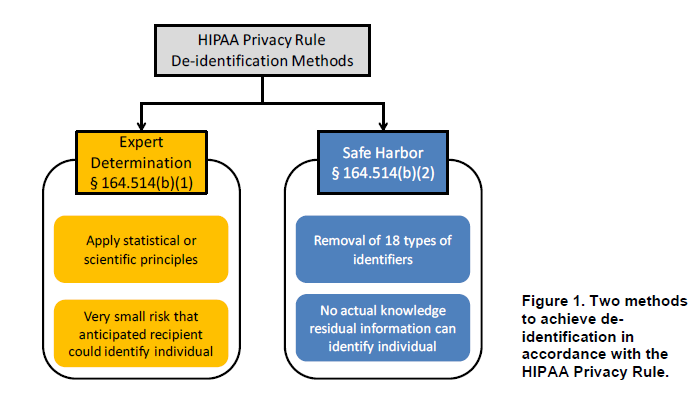 Source: HHS Guidance Regarding Methods for De-identification of PHI in Accordance with the HIPAA Privacy Rule
Source: HHS Guidance Regarding Methods for De-identification of PHI in Accordance with the HIPAA Privacy Rule